“In-stent restenosis is the main indication for DCB, and represents an especially challenging population. Though previous trials have shown very positive outcomes for DCB in treating in-stent restenosis1, little was known as to whether lesion preparation would have any influence on the outcome,” commented clinical investigator Dr. Robert Byrne, German Heart Center, Munich, Germany. “ISAR- DESIRE 4 further establishes DCB as a valuable tool to treat in-stent restenosis, independent of the type of lesion preparation.”
ISAR-DESIRE 4 randomized 250 patients into two study arms; after lesion preparation with either standard uncoated balloon predilatation or SCB, both groups were treated with Pantera Lux. The primary endpoint was percent diameter stenosis (%DS) at six to eight months. Though primary endpoint outcomes for the SCB group were slightly improved (35%DS v. 40%DS, p=0.047), DCB treatment with Pantera Lux effected overall positive clinical outcomes for both.
“In the most challenging cases seen in this trial, it appears that Pantera Lux’s performance can be even further enhanced by using SCB predilatation,” stated Dr. Byrne. “These benefits are likely caused by better drug uptake due to micro-injuries created by the scoring balloon.”
“Pantera Lux has established itself in both clinical trials2,3 and routine clinical practice as a highly safe and efficacious treatment for in-stent restenosis, regardless of whether the lesion is first prepared with a scoring balloon,” commented Dr. Alexander Uhl, Vice President Marketing, BIOTRONIK Vascular Intervention. “These results offer further confirmation that DCB is the current gold standard for treating in-stent restenosis without leaving another implant behind.”
About Pantera Lux
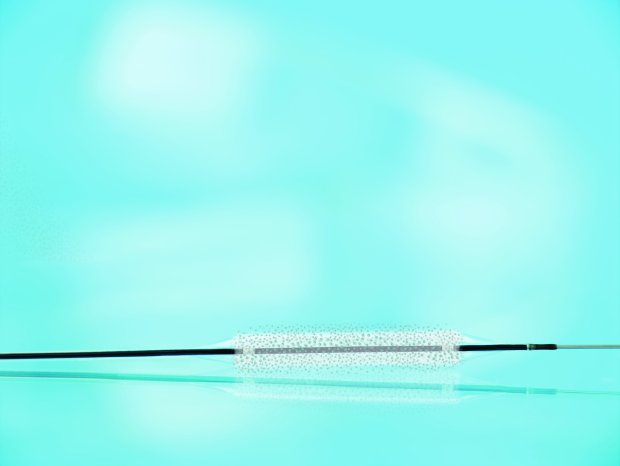
Pantera Lux is a clinically proven coronary drug-coated balloon, indicated for treating in-stent restenosis, de novo lesions, small vessels and acute occlusions. Based on the highly deliverable Pantera platform, the balloon is coated with a matrix of the proven anti-proliferative drug paclitaxel and the biocompatible excipient BTHC, enabling optimal drug transfer to the target lesion.
References:
1 Byrne RA, et al. Lancet. 2013, 381(9865)
2 Toelg R, et al. EuroIntervention. 2014, 10(5)
3 Hehrlein C, et al. Cardiovasc Revasc Med. 2012, 13(5)