Cardiovascular disease is the number one cause of death globally.2
Implantable cardioverter defibrillators (ICDs) control life-threatening arrhythmias, including those that cause sudden cardiac arrest. Some ICD patients also have heart failure, which affects 26 million people worldwide,3 or two to three percent of the adult population.
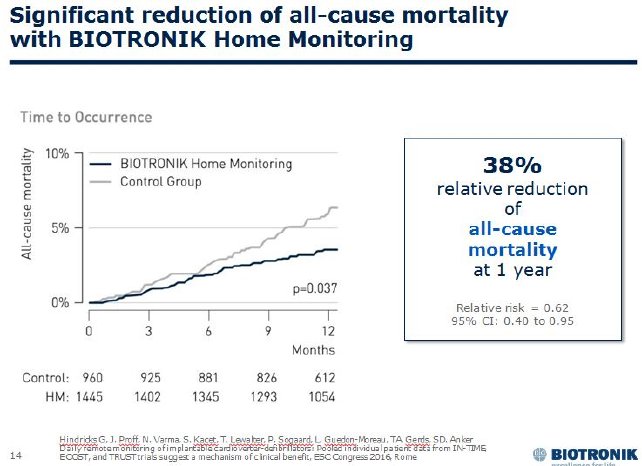
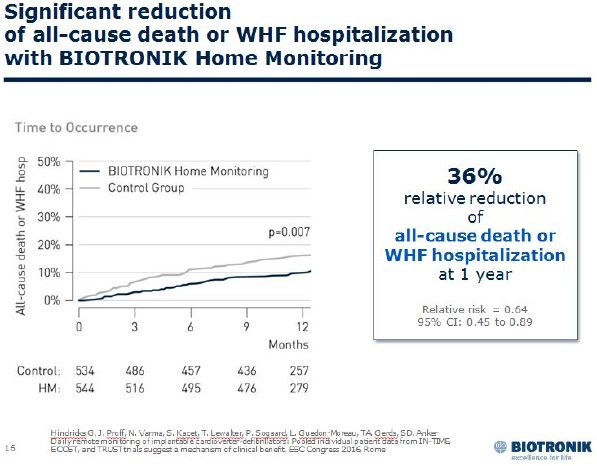
The TRUECOIN study pooled 2,405 patients from the TRUST4, ECOST5 and IN-TIME6 trials. The IN-TIME study showed more than a 50 percent risk reduction in all-cause mortality in ICD and CRT-D (cardiac resynchronization therapy defibrillator) patients with symptomatic heart failure. Based on these results, the European Society of Cardiology (ESC) recommends remote monitoring with the IN-TIME approach for this patient group in its latest heart failure guidelines.
The new meta-analysis extends the observed mortality benefit to a broader range of ICD patients with less severe heart failure. Furthermore, TRUECOIN identified the prevention of heart failure exacerbation as the underlying clinical mechanism of the observed benefits. Daily automatic transmission and analysis of a multiparameter data set make it possible to detect changes in heart failure status.
Other clinical studies like REM-HF, presented at ESC 2016, investigated remote monitoring systems and devices from other manufacturers, and have not found clinical benefits with remote monitoring. Dr. Gerhard Hindricks, lead investigator of TRUECOIN and IN-TIME, as well as president of the European Heart Rhythm Association, explained why TRUECOIN and IN-TIME show mortality benefits while other studies have not.
“It matters which technology you use,” commented Dr. Hindricks, Leipzig Heart Center, Germany. “The IN-TIME and TRUECOIN results show that BIOTRONIK Home Monitoring leads to improved clinical outcomes for patients. I believe this is due to differences in manufacturers’ technology, the data collected and clinic workflow.”
Concurrent with the publication of the TRUECOIN results, another study by Varma et al. investigating battery longevity and transmission reliability of Home Monitoring devices was published in Europace. The TRUST post-hoc analysis shows that Home Monitoring conserves battery longevity despite daily data transmissions. Results endorse the Heart Rhythm Society (HRS) Class 1A recommendation that remote monitoring be employed for battery conservation. The study also finds daily Home Monitoring transmissions to be highly reliable.7
About BIOTRONIK Home Monitoring
BIOTRONIK Home Monitoring® can be programmed by a physician to transmit data automatically and on a daily basis, thereby rapidly detecting deterioration in a patient’s clinical status. The occurrence of atrial or ventricular arrhythmias or specific trends in certain clinical parameters can often be the first sign of worsening heart failure leading to hospitalization or death. Early detection of these clinically relevant events, in particular asymptomatic atrial fibrillation, enables the physician to adapt patient therapy at a very early stage.
References:
1 Hindricks G et al. European Heart Journal. 2017, May 10. doi: https://doi.org/10.1093/eurheartj/ehx015
2 WHO Cardiovascular Diseases Fact Sheet, 2016.
3 CDC Heart Failure Fact Sheet, 2016.
4 Varma N et al. Europace. 2011, 13 (3).
5 Guedon-Moreau L et al. European Heart Journal. 2013, 34 (8).
6 Hindricks G et al. Lancet. 2014, 384 (9943).
7 Varma N et al. Europace. 2017, May 10. doi: https://doi.org/10.1093/europace/eux059