"The ultra-thin strut PRO-Kinetic Energy stent has a low profile and is extremely deliverable," commented national principal investigator for the study Dr. Saurabh Gupta, Director of the Cardiac Catheterization Laboratory at Oregon Health & Science University, Portland, Oregon. "I look forward to this innovative technology being available to all of my patients requiring treatment with a bare metal stent."
The BIOHELIX-I prospective, non-randomized, multi-center study (NCT01612767) has enrolled 329 patients in the US, Europe and South America. The primary endpoint for the study is the rate of target vessel failure nine months after stent implantation, a composite endpoint encompassing cardiac death, myocardial infarction and ischemia-driven target vessel revascularization.
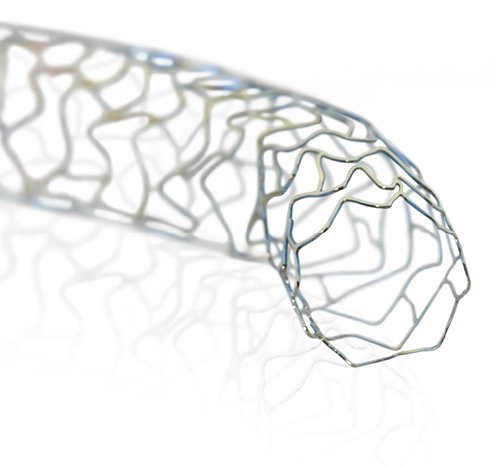
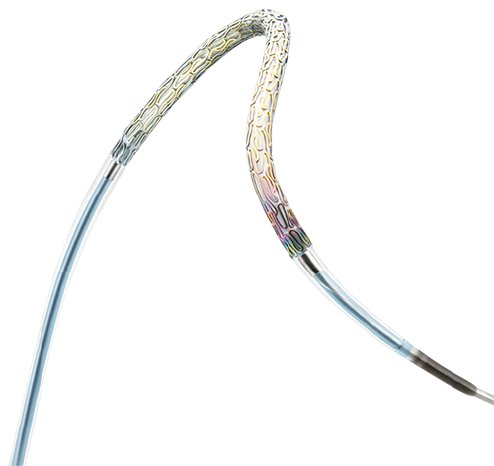
PRO-Kinetic Energy is an ultrathin strut (60μm / 0.0024") cobalt chromium, bare metal stent, completely sealed with a thin layer of amorphous silicon carbide, called proBIO. This passive coating is known to reduce metal ion release from the stent, limiting adverse events post-implantation.
PRO-Kinetic Energy was previously evaluated for safety and effectiveness in the ENERGY registry, an all-comers registry, which studied more than 1,000 patients in Europe and Israel. The ENERGY registry studied more than 1,000 patients with a complex population of 39% B2/C lesions and 46% acute coronary syndrome patients. At 12 months, PRO-Kinetic Energy demonstrated a low 8.8% major adverse cardiac events (MACE) rate, including a 3.4% rate of target lesion revascularization (TLR). A sub-group analysis showed similarly low 12-month MACE rates in acute coronary syndrome (ACS) patients, elderly patients, and patients with small vessels or diabetes.
"The PRO-Kinetic Energy stent has already proven itself as an excellent treatment option for over half a million patients," said Dr. Daniel Buehler, President, BIOTRONIK Vascular Intervention. "The BIOHELIX-I trial brings BIOTRONIK one step closer to achieving its goal of offering US physicians a complete portfolio of innovative solutions for treating coronary disease."
About PRO-Kinetic Energy*
The PRO-Kinetic Energy bare metal coronary stent features ultrathin struts of only 60 µm and a unique double-helix design, resulting in exceptional flexibility and deliverability even in challenging vessels. The stent is made from a cobalt chromium alloy and is coated with proBIO, a silicon carbide layer that reduces metal ion release from the stent surface into the surrounding tissue. The PRO-Kinetic Energy Stent platform also forms the basis of the Orsiro Hybrid Drug-Eluting Stent** and the PK Papyrus** stent system for treating acute coronary artery perforation.
* CAUTION - Investigational device. Limited by United States law to investigational use.