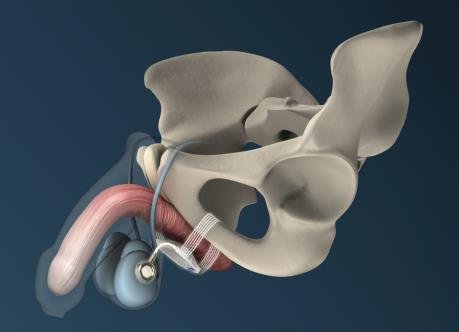
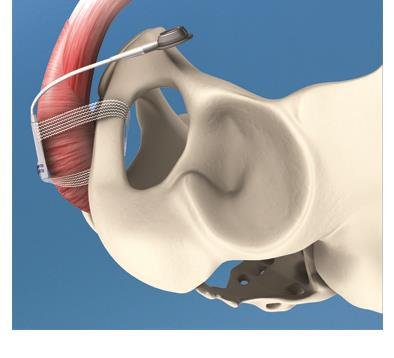
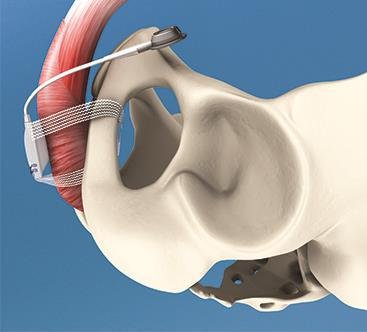
Designed to allow post-operative adjustment without surgical reintervention, the original ATOMS system comprises a small silicone cushion placed under the bulbar urethra and a coin-sized access port for adjusting the volume of liquid in the cushion. Up until now, this access port has been placed subcutaneously in the suprasymphyseal region. Presented by medical device manufacturer A.M.I. for the first time at the 28th Annual Congress of the European Association of Urology held recently in Milan, and currently on display at the American Urological Association's Annual Meeting in San Diego, the new access port is designed for placement in the scrotum instead. This development means even less invasive surgery and a high level of convenience, without compromising the proven performance of the existing device. Since its introduction to the market some four years ago, the A.M.I. ATOMS System has been implanted by urologists around the globe and has achieved high rates of both continence and patient satisfaction worldwide[1],[2],[3].
Excellent track record
Data published over the last 12 months includes very positive results from 3-year follow-up, which show a success rate of more than 90% for a patient population with severe incontinence[1]. In addition, secondary radiation therapy appears to be no contraindication for treatment with ATOMS, with data showing the same outcome for both irradiated and non-irradiated patients[3]. There is little doubt that these very promising results are due in no small part to the system's adjustability - the option of being able to alter pressure on the bulbar urethra at any time post-operatively allows the surgeon to find the ideal balance for each individual patient. This ability to fine tune the system via the access port with no surgical reintervention represents a significant advantage over other systems and considerably lowers the risk of infection, which is present every time surgery is performed with a prosthetic device.
As explained by Dr. Wilhelm Bauer, a leading Viennese urologist and ATOMS expert, the system is self-anchoring and does not require any bone screws or other means of fixation to secure it in the pelvis. Then, with the system in place, "the patient is able to urinate freely without having to activate a mechanical component". This concept of physiological voiding is particularly relevant for elderly patients, for whom manual operation of a system can be especially challenging.
The single-incision advantage
By adding the option of a port for scrotal placement, A.M.I. has raised the level of comfort for both surgeon and patient even further. All the system components can now be implanted through just one incision, not only saving operating time but also further reducing the risk of infection[1],[2],[3]. In addition, the single-incision approach means fewer scars for the patient and a lessening of foreign-body sensation in the suprasymphyseal region. With the single-incision approach rapidly gaining popularity amongst surgeons and patients alike, A.M.I.'s Director of Sales & Marketing, Marc Jablonowski, says ATOMS is just one of several surgical procedures that has been further developed to allow for single-incision use. Not only do patients reap the cosmetic benefits; regardless of the procedure they are also likely to experience less discomfort due to fewer incisions. This may mean a quicker recovery and shorter hospital stay, which in turn lowers the cost for healthcare facilities.
Clinical advantages of ATOMS with scrotal port:
- Considerably lower operating time
- Only one incision, therefore less risk of adverse events during surgery including a lower risk of infection
- One less scar
- Minimises suprasymphseal foreign-body sensation for patients
Advantages of ATOMS
- Post-operatively adjustable with liquid injected through the access port, no surgery is required
- Physiological urination
- Suitable for low to high grade incontinence[2]
- Results comparable for radiated and non-irradiated patients[3]
[1]. Hoda MR, Primus G, Schumann A et al.
Behandlung der Belastungsinkontinenz nach radikaler Prostatektomie: Adjustierbares transobturatorisches System - prospektive multizentrische Anwendungsbeobachtung Urologe A. 2012 Nov;51(11):1576-83.
[2].Hoda MR, Primus G, Fischereder K et al.
Early results of a European multicentre experience with a new self-anchoring adjustable transobturator system for treatment of stress urinary incontinence in men BJU Int. 2013 Feb;111(2):296-303
[3].Seweryn J, Bauer W, Ponholzer A, Schramek P.
Initial experience and results with a new adjustable transobturator male system for the treatment of stress urinary incontinence. J Urol. 2012 Mar;187(3):956-61