High on safety, low on pain
With no major intra- or post-operative complications recorded and an overall complication rate of 8%, the study’s findings are in line with those presented in other publications, which show HAL-RAR to be a very safe option for surgical treatment of haemorrhoids. While the design of this study did not include an assessment of post-operative pain, results also showed a very low degree of discomfort, with only one patient suffering from persistent pain for more than 4 days after surgery. Particularly when compared with other surgical procedures, which may result in severe pain and in some instances quite serious complications, HAL-RAR appears to live up to its reputation as the gentle treatment option.
Superior relief from symptoms
In terms of efficacy, the results achieved for control of pain, pruritus and bleeding were superior to those published for Conventional Haemorrhoidectomy and Stapled Haemorrhoidopexy, with almost 90% of patients being symptom free at 12 months. The re-prolapse rate was slightly higher than that published for CH, however the authors note that recurrence of prolapse may not always be the main concern for all patients. While most studies for higher grades of haemorrhoids tend to focus on this aspect, Professor S. Roka and co-authors of this study are of the opinion that both patient satisfaction and “control of symptoms other than prolapse .... are more relevant in evaluating efficacy”.
The significance of ligations
For the first time, it was also shown that the effectiveness of the procedure is due in no small part to the ligation of haemorrhoidal arteries. According to the multivariate analysis, the number of ligations was the factor having the most statistically significant impact on the recurrence of symptoms. Optimal results were shown to be achieved with 5 – 7 ligations and 3 – 4 prolapse reduction sutures. In addition, the number of ligations was the only factor showing any significant correlation with patient satisfaction. The number of prolapse reduction sutures was also shown to influence the recurrence of symptoms, although not as significantly as the number of ligations. According to A.M.I.’s Sales & Marketing Director, Marc Jablonowski, these findings are the very first of their kind and provide scientific proof of the importance of HAL, even in treating high grades of haemorrhoids. “There can no longer be any doubt that the accurate ligation of a sufficient number of targeted haemorrhoidal arteries plays a significant role in the effective treatment of haemorrhoids.”
The authors conclude that the HAL-RAR methods are both safe and effective, with a high degree of patient satisfaction. Based on the “perioperative benefits, very low number of complications, good symptom control and acceptably low recurrence rate”, the centres contributing to the study have chosen HAL-RAR as their first-line treatment for high-grade haemorrhoids.
Key Data
- 184 patients
- 58% Grade III; 42% Grade IV
- Operating time (mean): 35 mins
- Number of ligations: 5 - 7
- Number of prolapse reduction sutures: 3 - 4
- Follow-up at 3 and 12 months
Key Results
- No major complications
- Low post-operative pain (VAS score)
- 89.4% symptom free at 12 months
- 84.4% symptom and prolapse free
- Patient satisfaction equal for Grades III and IV
http://link.springer.com/...
About HAL-RAR
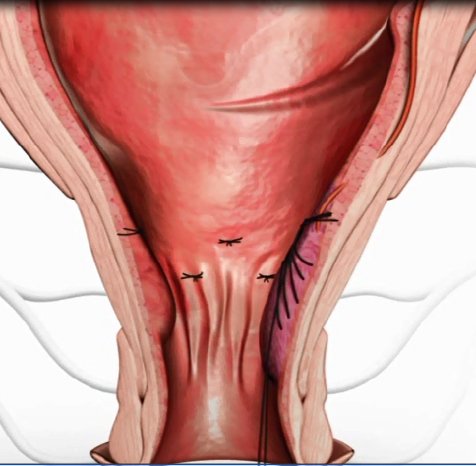
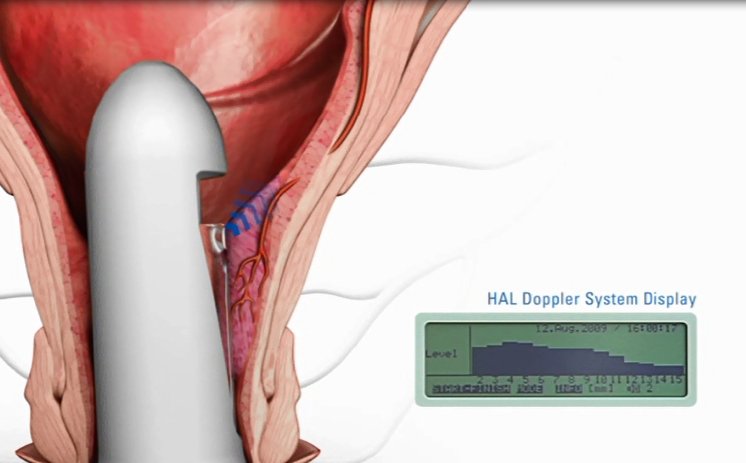
HAL (Haemorrhoidal Artery Ligation), a method offered by A.M.I., is used to stop the blood supply to the haemorrhoidal cushions. A miniature Doppler ultrasound device is gently inserted into the anus, and an audible signal allows the surgeon to pinpoint the exact location of the arteries. The surgeon then ligates each artery by placing a stitch around it and knotting the ends. Because the stitch is placed not in the anus but in the lower rectum, where there are almost no pain nerves, the whole procedure is virtually painless. With the blood supply to the haemorrhoids being obstructed, the pressure in them is reduced almost immediately and they start to shrink. In just a few weeks, they are no longer noticeable and the symptoms resolve. Where necessary - for example in the case of Grade III or IV haemorrhoids - RAR can also be used to reposition the prolapsing haemorrhoids.
The principle of RAR – Recto Anal Repair - is ingeniously simple. Using the same device as for HAL, a running stitch is made from top to bottom through the prolapsing tissue. By pulling the ends of the thread together and knotting them at the top, the surgeon is able to lift the tissue back up into place and secure it where it belongs. The area subsequently scars over and integrates seamlessly back into the anal tissue.
About A.M.I.
A.M.I. – Agency for Medical Innovations – is an Austrian manufacturer of medical technology based near the shores of Lake Constance, within easy reach of both Germany and Switzerland. For more than a decade we have been developing, producing and distributing our medical products from this location. Our activities are focussed on the areas of minimally-invasive surgery, coloproctology, bariatric surgery and urogynaecology.
One of the company’s main strengths lies in the fact that we develop and manufacture products according to the highest European standards of quality, and do so as quickly as possible. We pride ourselves on reducing to a minimum the time that elapses between a product idea and its realisation.
This strength has allowed A.M.I. to experience continual growth, and to gain a firm foothold in the market as an established European manufacturer of medical products. We develop products with that special something extra and have set a trend on several occasions. Despite their differing applications, our products all have one thing in common - based on innovative ideas and manufactured according to our high quality standards, they enable doctors to take even better care of their patients.